Projects supported by Neurochlore
Combined Approach to Treating Brain Tumors
Context
Despite massive investments and major advancements, there is still no truly effective treatment for brain tumors. Conventional approaches, such as chemotherapy and radiation therapy, only slightly prolong patient survival, often at the cost of severe side effects. As for emerging strategies, such as Tumor Treating Fields (TTF) therapy or immunotherapy, they remain expensive, uncertain, and difficult to generalize, posing a major financial challenge, particularly for healthcare systems and low-resource countries.
One of the fundamental reasons for these failures lies in the lack of consideration for the tumor microenvironnement, particularly the close relationship between the neuronal environment and the tumor.
Recent studies have shown that tumor cells establish functional synaptic connections with surrounding neurons, generating hyperactivity that promotes their proliferation and metastatic spread (Barron, Tara, et al. 2025). A key player in this phenomenon is the altered GABAergic polarity in tumor cells. Unlike mature neurons, where GABA is inhibitory, cancer cells accumulate abnormally high levels of intracellular chloride, mainly due to overexpression of NKCC1. This alteration transforms GABA into an excitatory signal, promoting tumor growth instead of inhibiting it.
Ignoring this complex dynamic, specific to brain networks, significantly limits the effectiveness of current treatments. Specifically targeting these neuronal interactions and restoring normal GABAergic polarity could provide an innovative and more effective approach to combat brain tumors.
Project
In response to this, Neurochlore has initiated a project proposing an innovative combined strategy, based on the synergistic action of two molecules: Bumetanide, an NKCC1 inhibitor aimed at blocking the neuronal hyperactivity associated with the tumor, and Mebendazole (MBZ), an antiparasitic agent known for its ability to target microtubules and induce the destruction of tumor cells. The anticancer potential of the latter has already been demonstrated in numerous experimental studies, as well as in a Phase I/II clinical trial on glioblastoma patients conducted by a team at Johns Hopkins University (Gallia, Gary L., et al., 2021).
Bumetanide (BUM)
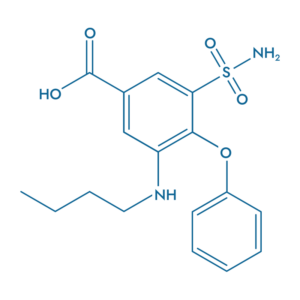
- Blocks hyperactivity
- Prevents metastasis
Mebendazole (MBZ)
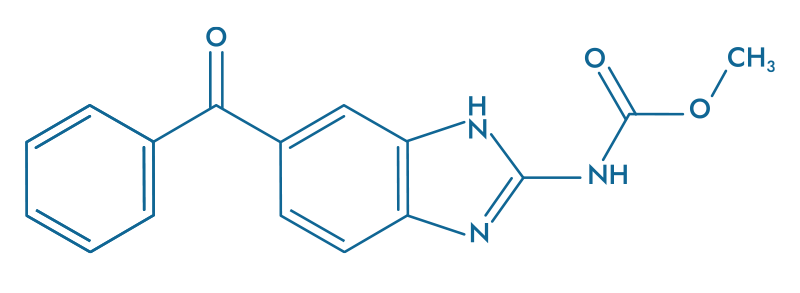
- Destroys microtubule
- Kills tumorals cells
By combining these two complementary approaches, our goal is to offer a more effective and better-tolerated treatment, acting not only on the tumor itself but also on its environment, which is key to its progression.
Leveraging the expertise of B&A Oncomedical in various ex vivo and in vitro applications, ranging from cell culture to advanced electrophysiological techniques, as well as fruitful collaborations with Prof. F. Berger and his team in Grenoble, our objective is to assess the potential of this therapeutic combination in the treatment of glioblastoma, a highly aggressive malignant brain tumor.
Given the distinct mechanisms of action of Mebendazole and Bumetanide, we have evaluated the effects of their combination on several experimental models.
Glioblastomas disrupt neuronal balance and promote synaptic hyperexcitability, leading to the onset of epileptiform seizures similar to those observed in epilepsy. Our research on freshly resected tumors from patients, recorded using the most advanced physiological techniques—including, for the first time, direct measurements of individual GABA channels and intracellular chloride (Cl⁻) levels—has allowed us to deeply explore these interactions. We demonstrated that Bumetanide, in the presence of Mebendazole, completely blocks epileptiform seizures in tumor cortical slices at low concentrations.
Results
Similar findings, where the combination of both drugs proved more effective than each molecule administered alone, were also obtained in “tumoroid” brain tumor models and in murine brain slices.
This synergy not only eliminates tumor cells but also significantly reduces neuronal hyperexcitability.
Furthermore, this interaction would allow for the use of lower doses of both compounds, thus minimizing potential side effects and improving treatment tolerance.
Finally, in the context of a compassionate pilot trial, the administration of the combination to one patient showed a remarkable increase in life expectancy.
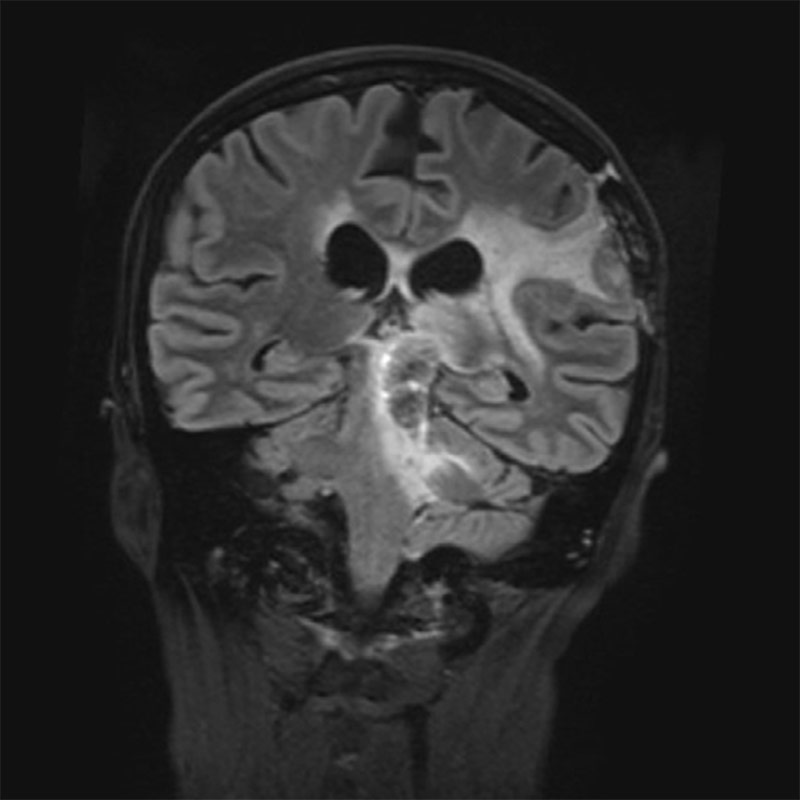
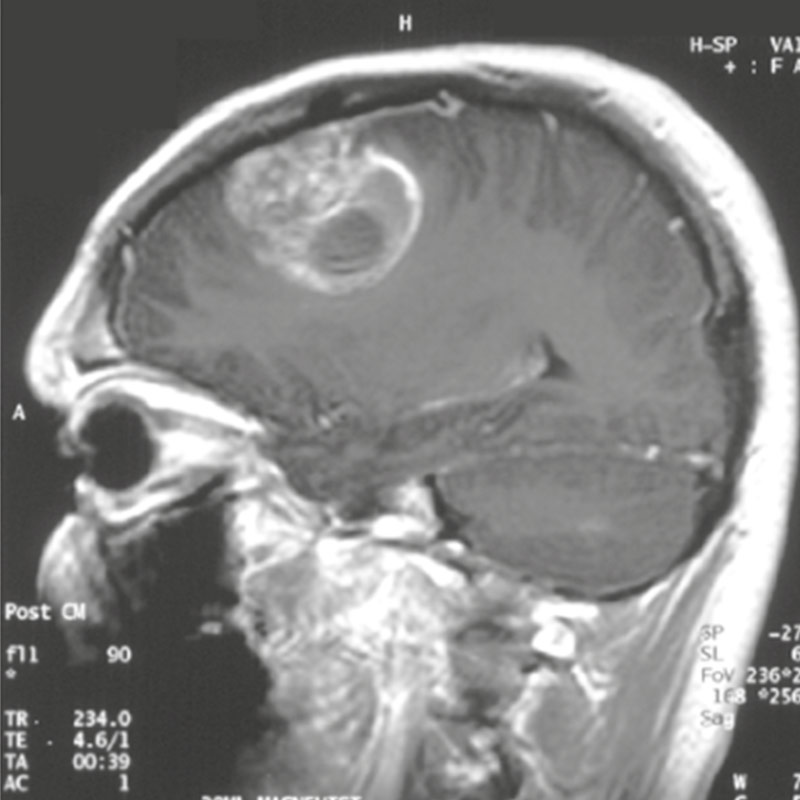
These promising results, covered by a PCT application, pave the way for an innovative therapeutic approach targeting both tumor cells and their neuronal environment, with potential applications for other brain tumors. Our goal now is to secure funding and mobilize investors to launch a Phase 2/3 clinical trial evaluating the effectiveness of the BUM x MBZ combination for the treatment of glioblastomas and other brain tumors.


