Projects supported by Neurochlore
Development of New NKCC1 Inhibitors: A Versatile Approach with Multiple Applications
Context
The NKCC1 transporter plays a key role in many pathophysiological processes, including neuronal hyperexcitability, neurodevelopmental disorders, and tumor growth. Its inhibition represents a promising therapeutic strategy with multiple applications, ranging from the treatment of Autism Spectrum Disorder (ASD) and epilepsy to combating brain tumors and other conditions where chloride homeostasis is altered.

In collaboration with world-renowned experts in the NKCC1 co-transporter and the Edelris laboratory, a leader in the development of new chemical compounds, Neurochlore has developed new NKCC1 inhibitors that are both more specific and more effective, with the goal of optimizing their therapeutic action while minimizing side effects.
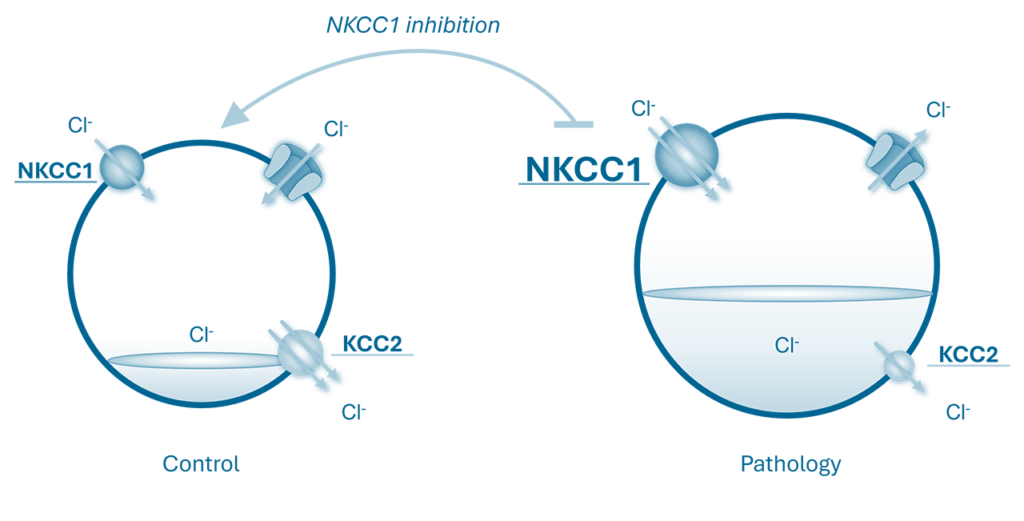
These new compounds, derived from Bumetanide — a known NKCC1 inhibitor with the ability to block neuronal hyperactivity by inhibiting this co-transporter — help restore low intracellular chloride levels, thus reinstating the inhibitory role of GABA.
Project & results
This partnership has resulted in 120 proprietary inhibitors of the NKCC1 transporter, protected by patents.
All these compounds were initially evaluated for their ability to inhibit NKCC1 in human cell lines. Most of them showed efficacy rates ranging from 75% to 100% compared to the reference molecule, Bumetanide. However, two compounds even surpassed it, achieving an efficacy of 120%. To our knowledge, no NKCC1 inhibitor on the market offers such promising results.
As part of our innovative approach for the treatment of brain tumors, which relies on a novel combination of Bumetanide and Mebendazole, these new compounds block hyperactivity without affecting intrinsic ionic currents. Even more surprisingly, the effectiveness of these compounds in inhibiting hyperactivity within the tumor environment is significantly enhanced in the presence of Mebendazole, known for inducing tumor cell death. Further analysis has shown that Mebendazole also acts as a partial inhibitor of NKCC1, suggesting that these compounds may not only act complementarily but also synergistically.
It is essential to highlight that the alteration of chloride homeostasis linked to the NKCC1 transporter is involved in numerous pathologies, and several studies have suggested significant improvements following its inhibition.
For example, in Alzheimer’s disease, recent research has demonstrated that long-term use of Bumetanide reduced the risk of developing the disease by a factor of three, based on data collected from 5 million American citizens over the age of 65 (Taubes, Alice, et al. 2022). Indeed, Bumetanide has been referred to as the “most promising treatment for Alzheimer’s” among more than 1,000 widely used agents.
The discovery of these new NKCC1 inhibitors thus represents a major breakthrough, potentially paving the way for a therapeutic revolution by targeting fundamental mechanisms involved in many neurological and oncological diseases.
Building on these promising results, our current goal is to select the five leading innovative NKCC1 antagonists and conduct the preliminary studies for the IND, which will lead to a Phase 1 trial, aimed at treating glioblastoma and other brain tumors, as well as a wide range of other diseases.


