Projects supported by Neurochlore
Using artificial intelligence to identify responders to Bumetanide
Yesterday…
Several independent clinical trials, as well as our two Phase II studies conducted on over 120 children with Autism Spectrum Disorder (ASD) (Lemonnier et al., 2012; 2017), have evaluated the effectiveness of Bumetanide to ameliorate the symptoms of ASD. These studies highlighted positive effects, reinforcing the potential of this molecule as a therapeutic option. They were validated in 7 other trials conducted by teams from different countries, with over 1036 children successfully treated using this approach (Xiao, Hong-Li, et al. 2024).
Our articles on phases II.a and II.b:
Based on these encouraging results, Neurochlore, in collaboration with Servier Laboratories, launched a Phase III study approved by European authorities. The goal was to confirm the effectiveness of Bumetanide in treating ASD and to prepare a market authorization application in Europe.

Details of the phase III studies are available on the following websites:
- For the study of children aged between 2 and 7 years: https://clinicaltrials.gov/ct2/show/NCT03715153
- For the study of children aged from 7 to 18: https://clinicaltrials.gov/ct2/show/NCT03715166
However, the results of this Phase III study did not show a significant difference between the treated group and the placebo group. The clinical evaluation criteria did not meet the required threshold, preventing the validation of the study and the market authorization of the treatment.
To date, all Phase III clinical trials for neurodevelopmental disorders have failed, largely due to the significant heterogeneity of the disorders, the diversity of patient profiles, and the complexity of these conditions.
ASD is characterized by a high inter-individual clinical heterogeneity, both in terms of symptoms and severity. This spectrum includes a wide range of manifestations, such as difficulties in social interactions, communication, and behavioral disturbances. Moreover, the presence of comorbidities, such as epilepsy or anxiety disorders, further increases this variability, complicating the evaluation of treatments.
Although the Phase III study did not show a significant benefit for the entire patient population, Bumetanide remains a promising therapeutic avenue for ASD. The results from Phase II, feedback from clinicians, and statistical analyses suggest that certain subgroups of patients may benefit from the treatment. (Read information note on the re-use of health data)

… Today
In this regard, Neurochlore has initiated a project aimed at identifying potential responders within the Phase III population, to better understand the factors influencing the effectiveness of Bumetanide and optimize its future use.
Building on B&A Biomedical’s expertise in clinical data analysis and artificial intelligence, we have reanalyzed the Phase III data, leveraging new computational methods in machine learning. The goal is to identify potential “Responders,” for whom the evaluation criteria meet the required thresholds and who show a significantly improvement with Bumetanide compared to the placebo group.
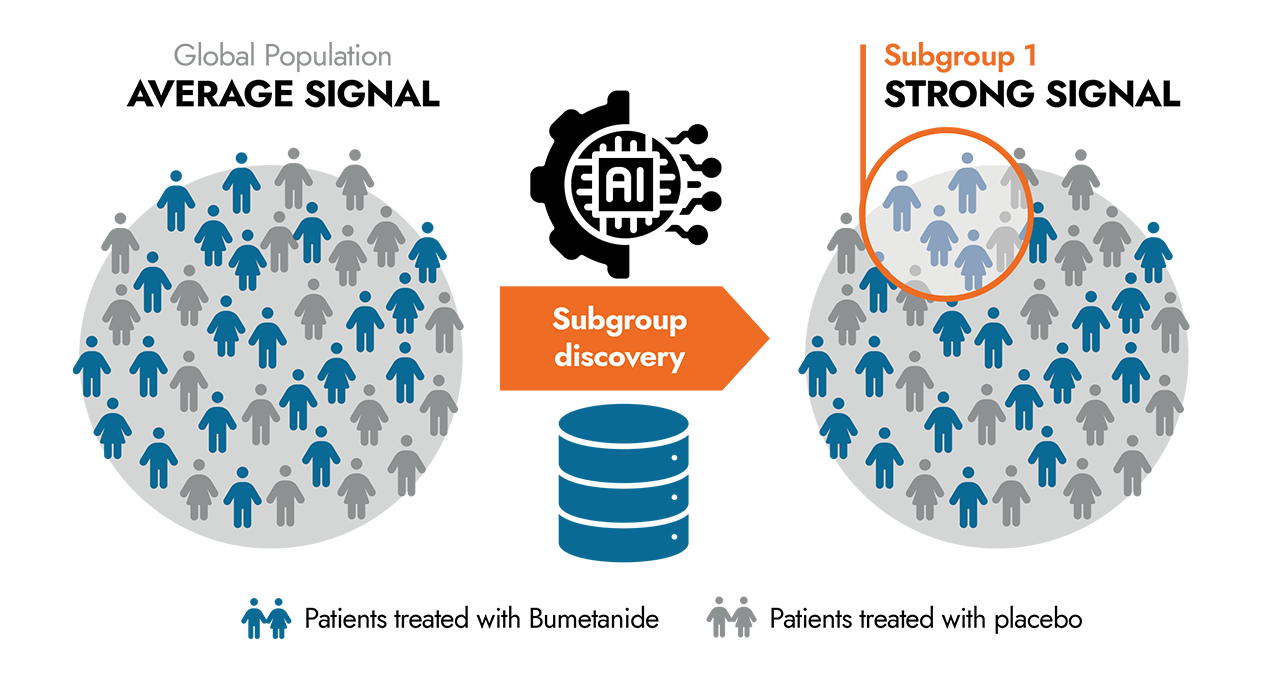
This approach has allowed us to identify subgroups showing a positive response to the treatment in approximately 30 to 40% of the population in this trial.
These patient subgroups are defined by clinical criteria related to the evaluation scales of these neurodevelopmental disorders.
Encouraged by these promising results, we are actively pursuing fundraising and investment opportunities to launch a new targeted study based on optimized inclusion criteria. This study, involving a newly recruited population of approximately fifty subjects selected according to these refined criteria, aims to validate our findings before initiating a new clinical trial—an essential step toward bringing the first autism treatment to market.
Beyond this study, this innovative approach provides a fresh perspective on optimizing clinical trials, with potential applications to other complex conditions.

